
En étroite collaboration avec l’ensemble des acteurs de la santé (pharmaciens, laborantins, médecins, établissements hospitaliers…), nous développons des dispositifs rapides, simples, pratiques qui facilitent la prévention, le dépistage et la mise en œuvre de traitements médicaux adaptés aussi bien aux besoins du patient qu’à celui du professionnel de santé.
Nous travaillons avec des instituts de renoms qui nous permettent de confronter nos équipes à la réalité du terrain et aux problématiques actuelles pour pouvoir concevoir des dispositifs toujours plus précis et plus fiables.

Approfondir le champ du diagnostic rapide, pousser plus loin la recherche, développer de nouveaux dispositifs sont nos préoccupations premières. Pour y répondre nous n’hésitons pas à investir dans des outils de pointe qui nous permettent d’être encore plus performants. Nous attachons une attention particulière à la qualité de nos produits qui passent entre les mains d’experts pour valider leur conformité aux normes et exigences requises.
Biosynex, c’est plus de 550 collaborateurs au service de la santé de tous.
Une entreprise certifiée: ISO 13485: 2016.
Les produits pour lesquels Biosynex est fabricant légal répondent aux exigences de la directive 98/79/CE et en transition au règlement européen EU2017/746 pour les DMDIV (Dispositifs médicaux de diagnostic in vitro) et au règlement européen EU2017/745 pour les DM (Dispositifs Médicaux).
Products for which Biosynex is a legal manufacturer meet the requirements of Directive 98/79/EC and in transition to European regulation EU2017/746 for IVDs (InVitro Devices) to European regulation EU2017/745 for MDs (Medical Devices).

biosynex
Maîtriser la chaine de valeur en france

Innovation
Biosynex met à disposition de chaque réseau de distribution (laboratoires de biologie, d’analyses médicales, hôpitaux, pharmacies, grandes surfaces…) une large gamme de produits adaptés. Elle oriente ses choix de développement et de distribution vers des niches de taille mondiale, spécifiques du marché, avec des produits maison à forte valeur ajoutée (TDR, santé féminine, suivi de traitement et aide médicale …). Afin de maintenir sa position, Biosynex a opté pour une politique de protection de la propriété intellectuelle volontariste en déposant ses propres brevets ce qui lui garantit son rôle d’acteur innovateur dans le secteur de la biologie.

nous connaître
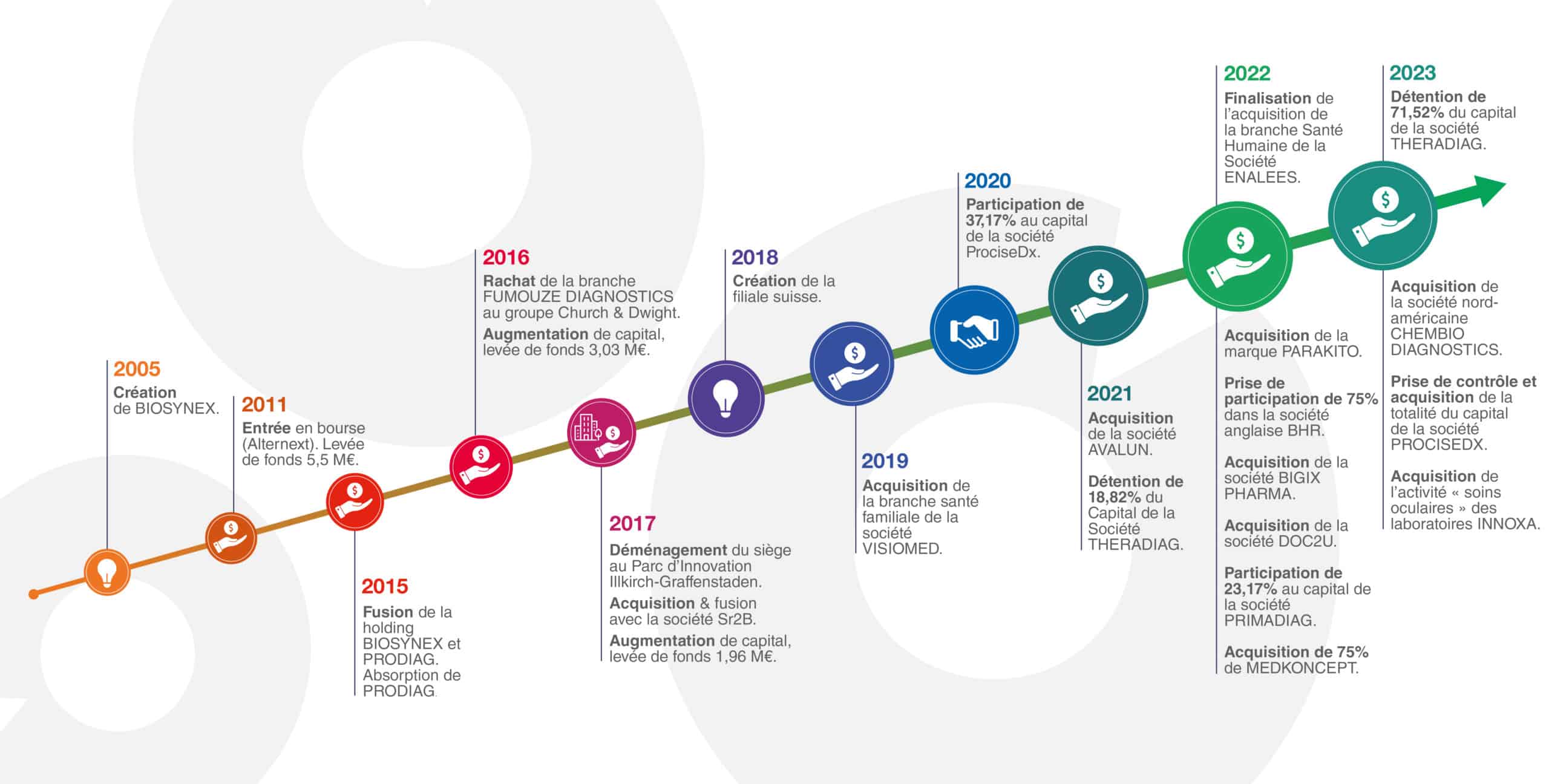
Notre histoire
Un groupe international
Nos structures

Diversité Femmes-Hommes

Promouvoir la diversité et l’égalité entre femmes et hommes est primordial pour BIOSYNEX. Vous trouverez ci-dessous les résultats de notre troisième index de l’égalité Femmes-Hommes*. BIOSYNEX obtient la note de 85/100. Ce résultat témoigne de la politique de diversité et d’égalité professionnelle mise en place au sein de notre entreprise, que ce soit au niveau des recrutements ou des conditions de travail.
Nous allons poursuivre nos efforts en faveur de la diversité Femmes-Hommes afin de maintenir et d’améliorer nos résultats dans les années à venir.
*Résultats obtenus en février 2025.

